RFK Jr.’s Confirmation Hearings Spotlight the Vaccine Safety Debate
This week, all eyes turn to Capitol Hill as Robert F. Kennedy Jr. faces confirmation hearings to lead an independent agency overseeing environmental and public health regulations. Known for his “controversial” stance on vaccine safety and the pharmaceutical industry, Kennedy has long been a polarizing figure in the health freedom movement. His vocal criticism of vaccine trials—particularly the lack of true placebo controls—has sparked both outrage and support, making these hearings a flashpoint for deeper conversations about accountability in public health policy.
If Robert F. Kennedy Jr. is confirmed to a position of influence over health and safety policy, his appointment would mark a seismic shift in the national conversation about vaccine safety. His confirmation offers an unprecedented opportunity to address these concerns head-on—but only if the Department of Health and Human Services (HHS) takes bold, corrective action.
A Call for a Moratorium to Restore Public Trust
The most effective way to restore public trust in childhood vaccines would be for HHS to implement an immediate moratorium on the current childhood vaccination schedule. Such a moratorium would not signal an end to vaccines but rather a temporary pause to re-evaluate their safety and efficacy using rigorous, transparent scientific methods.
A pause would allow for the following crucial actions:
1. Mandate Independent Safety Reviews
HHS should convene an independent panel of scientists, ethicists, and public representatives to reassess the safety profiles of all vaccines on the childhood schedule. This panel must operate free from pharmaceutical influence, with full public transparency.
2. Conduct True Placebo Trials
For the first time in decades, vaccines should undergo trials that include true inert saline placebos to determine baseline safety profiles. This is especially critical for combination vaccines, where cumulative effects are poorly understood.
3. Audit VAERS and Post-Market Surveillance Systems
A moratorium would also allow time to overhaul the Vaccine Adverse Event Reporting System (VAERS), automating data collection and mandating reporting by healthcare providers. With less than 1% of adverse events currently reported, VAERS cannot provide a reliable picture of vaccine safety.
4. Enhance Public Transparency
Every trial, every safety review, and every piece of adverse event data must be made fully accessible to the public. This level of transparency is the only way to address the growing skepticism fueled by decades of opaque processes.
If the manufacturers refuse to participate the liability protection they are afforded should be revoked.
Trust and Accountability in Public Health
As the nation debates whether Kennedy’s track record is an asset or a liability, the issue at the heart of his advocacy remains unresolved: How can the public trust vaccine safety when trial methods and data transparency leave so much room for doubt?
This is a lightning rod issue that has the rare ability to send otherwise rational people into childish, foot-stomping denials. It causes so much cognitive dissonance that it can ruin relationships. Objectively looking at the responses I’ve gotten from those who disagree, I understand why. Much like the COVID vaccine debates, this boils down to personal responsibility and the inability to accept that one may have made a terrible decision for themselves or their family. No one wants to admit they may have put loved ones in harm’s way.
The urge to deny this possibility often overrides rationality, triggering denial and anger directed at those who raise these concerns. These psychological defense mechanisms, long understood by behavioral scientists, have been weaponized against us. Governments and corporations use them to build consensus and marginalize dissent, while their desperation to control public opinion grows increasingly aggressive.
In the Information Age, dissenters are now being labeled “disinformation agents” or other pejoratives, with many voices being actively suppressed or even criminalized. This is why articles like this one are critical, and why individuals—not just journalists—must speak out if they value freedom. Waiting for the traditional media to address these issues is futile; they are fully integrated into a system of corporate and governmental collusion that operates like a neo-fascist state.
The Placebo Problem
When you hear the word “placebo” what comes to mind? Most imagine a harmless sugar pill or saline solution used as a neutral baseline in research. This fundamental principle ensures that drugs or vaccines are evaluated for their true safety and efficacy. Yet, for decades, vaccine trials have deviated from this standard.
Instead of saline placebos, vaccine manufacturers frequently use “active comparators”—often other vaccines or adjuvants that produce their own biological reactions. For instance, Merck’s HPV vaccine trials used an aluminum adjuvant as the placebo, masking potential side effects of the vaccine itself. A reanalysis of this data showed 2.3% of participants in both the vaccine and control groups experienced serious adverse events, creating an illusion of parity.
According to a BMJ review, only 31% of vaccine trials include any form of placebo control, and even fewer use inert saline placebos. This omission introduces significant blind spots, delaying recognition of adverse events like narcolepsy following the 2009 H1N1 vaccine in Europe.
Kennedy’s long history of challenging corporate influence and regulatory capture positions him uniquely to lead these reforms. The staggering increase in childhood vaccine doses—from 5 in 1963 to 72 today—demands a reckoning. A moratorium would create space for the transparency and accountability necessary to restore trust. Anything less risks perpetuating a broken system.
This moment is a once-in-a-generation opportunity for decisive leadership. If we fail to act now, the consequences will be far-reaching—not only for public health but for the very fabric of an informed, free society.
The Role of Regulatory Capture
The revolving door between pharmaceutical companies and regulatory agencies is another critical issue that undermines public trust. FDA officials frequently transition to lucrative roles within the very companies they once oversaw, creating an undeniable conflict of interest. This phenomenon, known as regulatory capture, is not limited to the FDA. We see a similar pattern in the media, where intelligence agency officials retire into high-profile positions at networks like CNN and MSNBC.
For example, Pfizer, which has repeatedly faced legal fines for improper marketing practices—including a record $2.3 billion settlement in 2009—continues to wield significant influence over regulatory decisions. To ignore this history of ethical violations and assume the industry’s intentions are always aligned with public health would be naïve at best and dangerous at worst.
The current system relies on pharmaceutical companies to self-report trial data, placing the fox firmly in charge of the henhouse. These corporations, with billions of dollars at stake, have every incentive to downplay adverse events or selectively report outcomes. Until the government takes a more proactive role in ensuring independent oversight, public skepticism will remain justified.
The Burden of Proof and the Placebo Debate
The vaccine industry’s reliance on active comparators rather than inert placebos highlights the deeper issue: an unwillingness to subject its products to the same rigorous testing demanded of other pharmaceuticals. This raises an uncomfortable question: If vaccines are as safe and effective as claimed, why avoid the gold standard of scientific research?
The ethical defense often cited—that withholding an effective vaccine from a control group would be harmful—falls apart under scrutiny. While it is true that some ethical dilemmas arise in placebo-controlled vaccine trials, they do not justify compromising the scientific integrity of safety assessments. The argument only serves to protect industry interests at the expense of public confidence.
To rebuild trust, public health authorities must embrace radical transparency. This includes mandatory public release of all clinical trial data, independent oversight of vaccine safety studies, and meaningful reforms to post-market surveillance systems like VAERS. Without these changes, the chasm between the public and those in power will only widen.
RFK Jr.’s confirmation represents a rare opportunity to confront these issues head-on. His critics argue that his positions are controversial or extreme, but they fail to acknowledge that the status quo is equally untenable. The question is no longer whether vaccine safety reform is necessary—it is whether the system has the courage to admit it.
A Call to Action
As I go through the reality of the lack of true safety studies conducted on vaccines administered to millions of children each year, ask yourself again: Why aren’t they using inert saline placebos if they are truly interested in understanding safety profiles?
The answer lies in a system that prioritizes expediency and profit over transparency and accountability. RFK Jr.’s confirmation hearings offer a pivotal moment to demand better—a chance to hold the pharmaceutical industry and its enablers accountable. If the government and its regulators truly want to rebuild trust, they must start by addressing the gaping flaws in vaccine trial methodologies.
This is not just about vaccines; it’s about the integrity of science, the accountability of government institutions, and the future of public health. The time for complacency is over. If we fail to act now, the cost will not just be measured in dollars but in the lives and trust we continue to lose.
The Childhood Vaccine Schedule Breakdown
Brand Name: Kinrix
Used For: (Diphtheria-Tetanus-Acellular Pertussis-Polio)
In the only trial specifically described in the package insert the control group received the Infanrix and polio vaccines. The package insert doesn’t mention any trial involving a placebo control group.

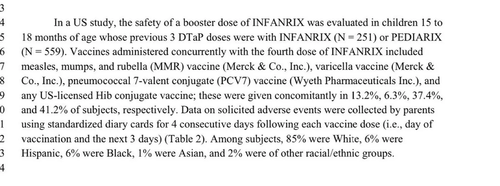
Brand Name: Infanrix
Used For: (Diphtheria-Tetanus-Acellular Pertussis)
Tested against a control group that received the DTP vaccine or no control group.

Brand Name: DTP
(Diphtheria-Tetanus-Pertussis)
The vaccine was developed in the 1930s and has never been tested in an RCT against a control group receiving a real placebo.

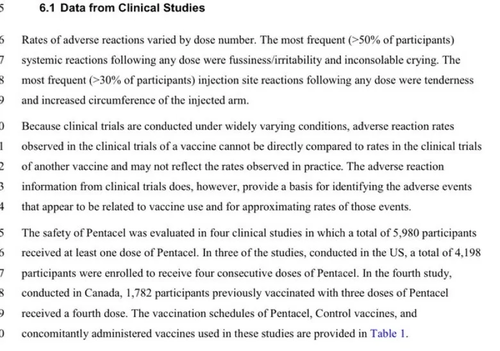
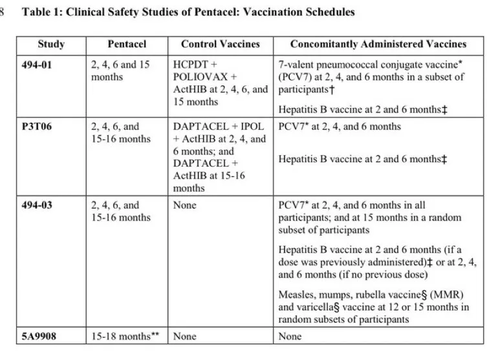
Brand Name: Pentacel
(Diphtheria-Tetanus-Acellular Pertussis-Polio-Hib)
The control groups in 3 of the 4 trials received an assortment of different vaccines. The 4th trial’s control group may have received no vaccines; however, its safety data is not presented in the package insert.
Pentacel FDA Clinical Safety Review
Brand Name: Quadracel
(Diphtheria-tetanus)
Tested against another vaccine

Brand Name: Prevnar
Pneumococcal disease
Tested against a control group that received an experimental meningococcal vaccine.

Brand Name: Prevnar-13
Pneumococcal disease
Tested against a control group receiving Prevnar (older-generation vaccine)

Brand Name: Engerix
(Hepatitis B)
Its side effect rate was compared to that of a previous generation product (plasma vaccine).
(Hepatitis A and B)
Tested in clinical trials against a control group that received separate hepatitis A and B vaccines.
(Hepatitis B Recombinant)
The package insert does not mention any safety RCT performed in infants.

Brand Name: Havrix
(Hepatitis A)
The control group in the main trial received the hepatitis B vaccine. In three other trials, the control group received several other vaccines (MMR, varicella vaccine, and more).

Brand Name: Vaqta
(Hepatitis A)
In one trial, there was no control group (according to another document, the control group received a compound that included aluminum and thimerosal), and in the second trial the vaccine was given concurrently with other vaccines and without a control group.
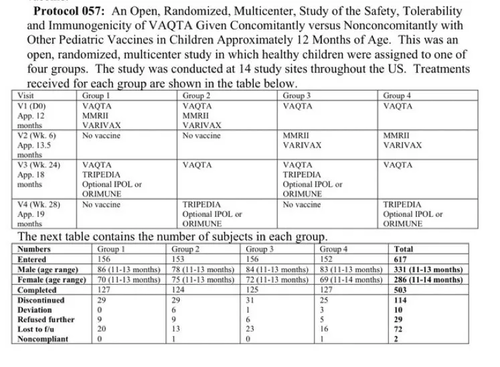
Brand Name: ProQuad
(Measles, Mumps, Rubella, Varicella)
Safety was tested in several randomized clinical trials, most of which were not blinded. None of the trials contained a control group receiving only a placebo.

Brand Name: MMR II
(Measles, Mumps, Rubella)
Tested in eight small unblinded clinical trials. All of the trials had one or more control groups receiving either the predecessor MMR vaccine, a measles-rubella (MR) vaccine, or a single-dose of the rubella vaccine.
MMR II FOIA Showing Lack of Placebo



Brand Name: MMR
(Measles, Mumps, Rubella)
Tested in several small to medium unblinded and partially randomized trials. The control groups totaled about 1/10 the number of subjects in the trial groups and received no injection.
237 Page MMR Clinical Trial Report
Brand Name: Varivax
(Varicella)
In one RCT the “placebo” given to the control group was actually the test vaccine from which the viral component was removed. Another trial compared two different formulations of the vaccine.
Study Describing Use of “placebo” with viral component removed.
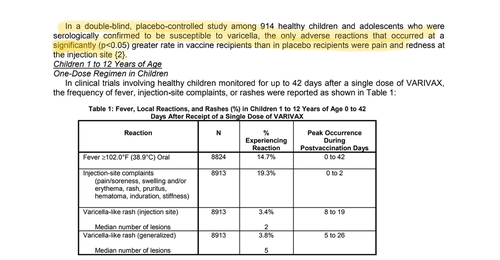
Brand Name: RotaTeq
(Rotovirus)
The control group in the trial probably received the vaccine-sans-antigen compound but the description of the control compound cannot be found in the FDA licensing documents. So we do not know for sure which is highly suspicious.
RotaTeq Efficacy & Safety Review
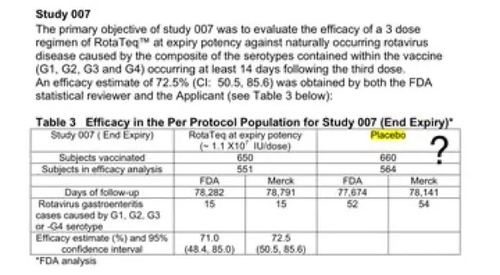
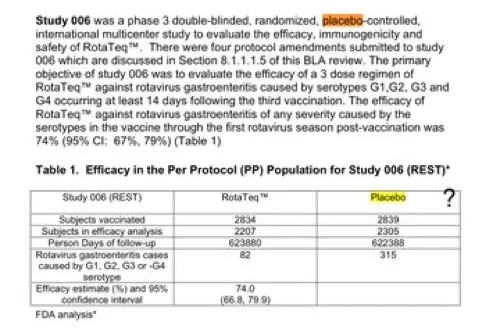
Brand Name: Rotarix
(Rotavirus)
The control group in the trial received the vaccine-sans-antigen compound.
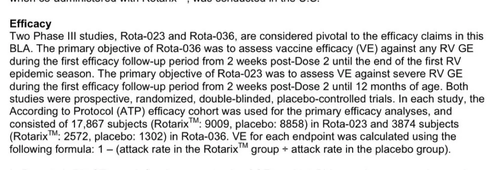

I’ve made this as straightforward as possible for you, providing direct links and screenshots so you don’t have to dig through dense inserts or obscure reports. Everything presented here comes straight from the source—whether it’s government data or the manufacturer’s own documentation. Now, let’s revisit the fundamental question: why would the FDA allow vaccines—intended for nearly every child in America—to be approved without testing them against a proper inert saline placebo? How can they possibly claim to understand the full safety profile when such a basic scientific standard has been ignored?
Moreover, why are vaccine manufacturers granted blanket liability protections, shielding them from the harms their products may cause, while being handed a perpetual, mandated market? What other industry in the world enjoys this level of protection, especially for products injected into newborn babies—the most vulnerable among us—without a second thought? And why, when a vaccine is pointed to as the cause of an injury, are doctors, media, and even friends and family culturally conditioned to immediately deny it?
This psychological sleight of hand is arguably one of the most pernicious deceptions ever perpetrated on the public. It is the result of relentless propaganda from cradle to grave—a drumbeat that has conditioned people to trust without question. The harsh reality is that many cannot reconcile the idea that they’ve been lied to their entire lives. Ego will often block even the slightest doubt from creeping in, no matter how much evidence of deceit surrounds them.
I hope, in some small way, this article can serve as a bridge to those in your life who have been programmed to trust liars and dismiss their critical-thinking loved ones as ‘conspiracy theorists’ or ‘extremists.’ At first, they may reject what you present, but challenge them to prove this information wrong. Ask them to find just one phase 3 clinical trial or manufacturer insert—used as the basis for the approval of any vaccine on the childhood schedule—that relied on an inert saline placebo to establish safety. Just one.
Remember, everything I’ve shared here is sourced directly from what the FDA used for approval and what manufacturers submitted for review. This isn’t conjecture or opinion—it is the stark reality. If they do not care that children are, in effect, being experimented on, then that is a matter between them and their conscience—or their maker.





